What Is the Oxidation State of a Lone Element
It is always 0. It is its most likely oxidation state.

The Periodic Table Of Oxidation States Oxidation State Teaching Chemistry Chemistry Lessons
If you now subtract the number of electrons assigned to H and O from their corresponding valence electrons you will.

. Oxidation states are something of an accounting fiction used to describe where electrons are spending most of their time in a molecule. The more electronegative the more the element attracts electrons. Oxidation state rule - 1 oxidation state of free atoms is zerofor example zns Als.
2 For monoatomic one atom species View the full answer. Match the element or group to the rule assigning its oxidation state. What is the oxidation state in lone elements and atoms in gases.
How do oxidation numbers relate to electron configuration Oxidation numbers are not so much related to electron configuration as they are to the polarity of the bonds to the atoms. Similarly oxidation state of diatomic molecule ie. The oxidation state of any lone element is zero.
The lowest oxidation state is 5 as for boron in Al 3 BC. The alkaline earth metals group II are always assigned an oxidation number of 2. Learn vocabulary terms and more with flashcards games and other study tools.
Rules 1 The oxidation number of the atoms in any free uncombined element is zero 2 The sum of the oxidation numbers of all atoms in a compound is zero 3 The sum of the oxidation numbers of all atoms in an ion is equal to the charge of the ion 4 The oxidation number of fluorine in all its compounds is 1. Lone elements and atoms in gases D. This is turn is dictated by electronegativity.
It is a number assigned to an element in a chemical compound that represent the number of electrons gained or lost by an element in a compound. What is the oxidation state of a lone element. Fluorine in compounds is always assigned an oxidation number of -1.
For a simple monoatomic ion the oxidation state is equal to the net charge on the ion. Start studying 531 Transition elements. The oxidation state of any lone element is zero.
So when you say oxygens oxidation state is -2 this is only with respect to other elements. General Rules Regarding Oxidation States. It is also called oxidation state.
In their compounds group-1 metals have an oxidation state of 1 In their compounds group-2 metals have an oxidation state of 2 4. The sum of the oxidation states of all atoms forming a molecule or ion is the net charge of that species. It is always 1.
How to compare heat of combustion of organic compounds. The fluorine always possesses the oxidation state -1 and it remains the same regardless of the nature of the compound it is a part of. For example Cl has an oxidation state of -1.
The oxidation number of a free element is always 0. So by the oxidation bookkeeping method oxygen is assigned a total of 8 electrons while hydrogen is assigned 0. Elements with multiple oxidation states E.
Oxidation number. Two atoms is also equal to zeroExamples- H2 Cl2 Br2 N2 O2. The oxidation number of a monatomic ion equals the charge of the ion.
The alkali metals group I always have an oxidation number of 1. Iron III oxide. Oxidation numbers are not so much related to electron configuration as they are to the polarity of the bonds to the atoms.
It could either accept or give. A part of hydrides since here the oxidation state of hydrogen will be -1. Rules for Oxidation Numbers.
Trinidadevelyn trinidadevelyn 11262018 Chemistry Middle School What is oxidation state for lone elements. The oxidation state of hydrogen is always 1 unless it is. In inorganic nomenclature the oxidation state is represented by a Roman numeral placed after the element name inside the parenthesis or as a superscript after the element symbol eg.
Helpful 0Not Helpful 0 Add a Comment. Any pure element has an oxidation state of zero. Consider a carbon atom for example.
The oxidation state of a free element uncombined element is zero. Have 2 atoms with lone pairs Other sets by this creator. Elements in groups 1 2 and 17 and polyatomic ions __c___ 0 ___E__ Ionic charge __B___ 1 1 if bonded to a diatomic metal ___a__ Almost always 2 __D__.
-Existence of more than one oxidation state in its compounds. Click here to get an answer to your question What is oxidation state for lone elements.

How To Know The Oxidation Numbers Of All The Elements By Group Quora
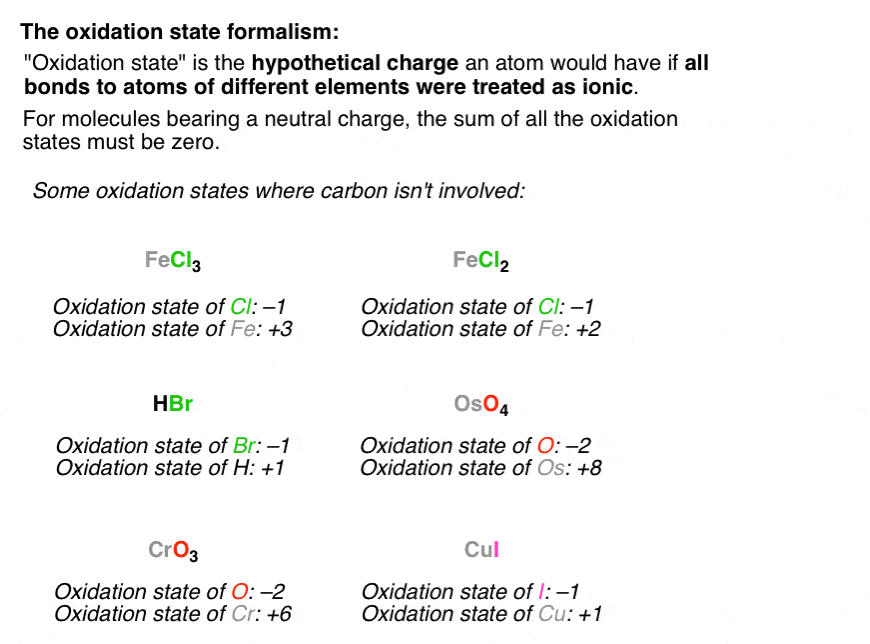
Calculating The Oxidation State Of A Carbon Master Organic Chemistry
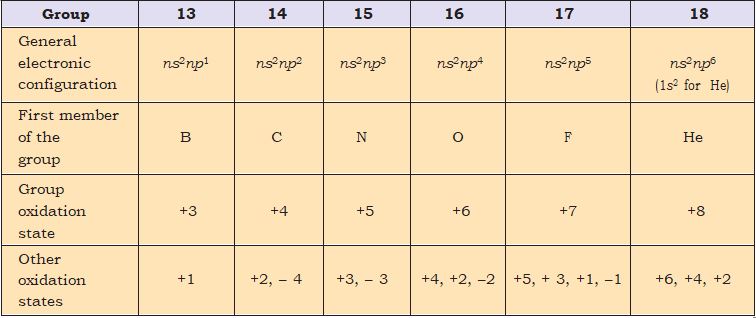
Comments
Post a Comment